I-gel Supraglottic Airway: Difference between revisions
Jump to navigation
Jump to search
(Created page with "==Procedure Guidelines== ===9.39 I-gel Supraglottic Airway=== I-gel is a second generation supraglottic airway, made of a medical grade thermoplastic elastomer, designed to c...") |
No edit summary |
||
| Line 10: | Line 10: | ||
*Epiglottic rest keeps the device from moving upwards out of position and prevents the epiglottis from down folding or obstructing the distal opening | *Epiglottic rest keeps the device from moving upwards out of position and prevents the epiglottis from down folding or obstructing the distal opening | ||
*Buccal cavity stabilizer a built in natural curvature and propensity to adapt its shape from the patient’s anatomy, the widened design also provides vertical strength and prevents rotation. | *Buccal cavity stabilizer a built in natural curvature and propensity to adapt its shape from the patient’s anatomy, the widened design also provides vertical strength and prevents rotation. | ||
[[File:iGels.jpg|framed|I-Gel]] | |||
'''INDICATIONS:''' | '''INDICATIONS:''' | ||
| Line 51: | Line 51: | ||
*Introduce the leading soft tip into the patient’s mouth of the patient in the direction of the hard palate. | *Introduce the leading soft tip into the patient’s mouth of the patient in the direction of the hard palate. | ||
*Glide the device downward and backward along the hard palate with a continuous but gentle push until definitive resistance is felt | *Glide the device downward and backward along the hard palate with a continuous but gentle push until definitive resistance is felt | ||
**Warning: Do not apply excessive force on the device during insertion. It is not necessary to insert fingers or thumbs into the patient’s mouth during the process of inserting the device. If there is early resistance perform “jaw thrust” to aid in insertion. | **Warning: Do not apply excessive force on the device during insertion. It is not necessary to insert fingers or thumbs into the patient’s mouth during the process of inserting the device. If there is early resistance perform “jaw thrust” to aid in insertion. | ||
[[File:Igel inserted.jpg|thumbnail|I-Gel Inserted]] | |||
*Once resistance is met and the teeth are located on the integral bite block, do not repeatedly push the i-gel down or apply excessive force during insertion. | *Once resistance is met and the teeth are located on the integral bite block, do not repeatedly push the i-gel down or apply excessive force during insertion. | ||
*NO more than three attempts in one patient should be attempted | *NO more than three attempts in one patient should be attempted | ||
Revision as of 19:49, 22 June 2016
Procedure Guidelines
9.39 I-gel Supraglottic Airway
I-gel is a second generation supraglottic airway, made of a medical grade thermoplastic elastomer, designed to create a non-inflatable anatomical seal of the pharyngeal, laryngeal and perilaryngeal structures. I-gel comes in four pediatric and three adult sizes available.
Key components and their functions:
- Soft non-inflatable cuff
- Gastric channel for regurgitation, which significantly reduces potential for regurgitation to get past the cuff and therefore aids in reducing the chance for aspiration. Please note size one i-gel does not have a gastric channel.
- Provides “vent” for gastric pressure and stomach decompression.
- Epiglottic rest keeps the device from moving upwards out of position and prevents the epiglottis from down folding or obstructing the distal opening
- Buccal cavity stabilizer a built in natural curvature and propensity to adapt its shape from the patient’s anatomy, the widened design also provides vertical strength and prevents rotation.

INDICATIONS:
- Airway management in pediatric cardiac arrest
- Inability to intubation when rapid control of airway is essential
- Difficult airway cases
- Passive oxygenation during cardiac arrest
CONTRAINDICATIONS:
- Responsive patient with intact airway –protective reflexes
- Patients with known esophageal disease
- Caustic ingestions
- Upper-airway obstructions due to foreign bodies or pathology
- Trismus, limited mouth opening, airway abscess, trauma or mass
Warnings:
- I-gel must be lubricated according to the instructions for use
- Patient should be in “sniffing” position, unless head/neck movement are contraindicated
- Leading edge of i-gel tip must follow the curvature of the patient’s hard palate upon insertion
- Excessive air leak during manual ventilations is a result of sub-optimal depth of the i-gel
EQUIPMENT:
- BVM
- Lubricant
- EtCO2 sensor
- Gastric tube
PROCEDURE:
- Size Selection:
- Select the appropriate size i-gel by assessing the patient’s anatomy. Although size selection on weight basis should be applicable to the majority of patients, individual anatomical variations mean the weight guidance provided should always be considered in conjunction with clinical assessment of the patient:
- Size 1: Neonate (wt. 2-5 kg)
- Size 1.5: Infant (wt. 5-12 kg)
- Size 2: Small Pediatric (wt. 10-25 kg)
- Size: 2.5: Large Pediatric (wt. 25-35 kg)
- Select the appropriate size i-gel by assessing the patient’s anatomy. Although size selection on weight basis should be applicable to the majority of patients, individual anatomical variations mean the weight guidance provided should always be considered in conjunction with clinical assessment of the patient:
- Apply small bolus of a water based lubricant to the distal tip and posterior aspect of the i-gel, taking care to avoid introduction of lubricant in or near the ventilatory openings.
- Pre-oxygenate via BVM.
- Suction upper airway if necessary
- Position the head. The ideal head position for insertion of the i-gel is the “sniffing position”. However, if there is failure to achieve complete insertion utilizing the standard insertion technique attempt insertion while performing a jaw thrust.
- Hold the i-gel with dominant hand, position device so that the i-gel cuff outlet is facing toward the chin of the patient. Non-dominant hand should gently press down on patients chin.
- Introduce the leading soft tip into the patient’s mouth of the patient in the direction of the hard palate.
- Glide the device downward and backward along the hard palate with a continuous but gentle push until definitive resistance is felt
- Warning: Do not apply excessive force on the device during insertion. It is not necessary to insert fingers or thumbs into the patient’s mouth during the process of inserting the device. If there is early resistance perform “jaw thrust” to aid in insertion.
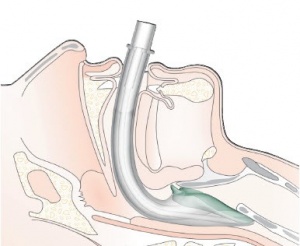
- Once resistance is met and the teeth are located on the integral bite block, do not repeatedly push the i-gel down or apply excessive force during insertion.
- NO more than three attempts in one patient should be attempted
- Attach BVM and Capnography to the 15 mm connector of the i-gel
- Secure i-gel, ensure that there is sufficient tension to hold the i-gel securely in place, but not excessive tension as to cause trauma to patients neck and or face or that may cause unwanted downward pressure on the i-gel causing displacement
- Ventilate patient at appropriate rate
- Confirm placement by auscultation and chest rise, monitor end title Co2
- For gastric distention, see chart below of appropriate nasogastric (NG) tube.
| I-gel Size | Maximum size of Nasogastric Tube |
|---|---|
| 1 | N/A |
| 1.5 | 10 |
| 2 | 12 |
| 2.5 | 12 |