ITClamp Hemorrhage Control System: Difference between revisions
Jump to navigation
Jump to search
(Created page with "==Procedure Guidelines== ===9.38 ITClamp Hemorrhage Control System=== '''Training:''' iTClamp™ hemorrhage control system requires specific training prior to use. '''INDICA...") |
mNo edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Procedure Guidelines== | ==Procedure Guidelines== | ||
===9.38 | ===9.38 iTClamp Hemorrhage Control System=== | ||
====TRAINING==== | |||
iTClamp™ hemorrhage control system requires specific training prior to use. | iTClamp™ hemorrhage control system requires specific training prior to use. | ||
====INDICATIONS==== | |||
The ITClamp™50 device is a trauma clamp device for the temporary control of severe bleeding in the extremities, axilla and inguinal areas. | The ITClamp™50 device is a trauma clamp device for the temporary control of severe bleeding in the extremities, axilla and inguinal areas. | ||
[[File:iTClamp.jpg|thumbnail|iTClamp device]] | |||
====CONTRAINDICATIONS==== | |||
The ITClamp50 is contraindicated where skin approximation cannot be obtained (for example, large skin defects under high tension). | The ITClamp50 is contraindicated where skin approximation cannot be obtained (for example, large skin defects under high tension). | ||
====WARNINGS==== | |||
*This device is intended for temporary
use only; FDA Approval is for 24 hours | *This device is intended for temporary
use only; FDA Approval is for 24 hours | ||
*Patients must be seen promptly by medical personnel for device removal and surgical wound repair | *Patients must be seen promptly by medical personnel for device removal and surgical wound repair | ||
| Line 19: | Line 20: | ||
*Ensure personal protective equipment is utilized to protect against potential splashing of blood during application | *Ensure personal protective equipment is utilized to protect against potential splashing of blood during application | ||
====PRECAUTIONS==== | |||
*Single-use, disposable device; not for reuse. Re-use of contents may cause cross contamination, leading to patient risk and complication(s). Can be repositioned. | *Single-use, disposable device; not for reuse. Re-use of contents may cause cross contamination, leading to patient risk and complication(s). Can be repositioned. | ||
*iTClamp50 is provided sterile (sterilized by EtO). Do not use if sterility seal has been tampered with or packaging is damaged. | *iTClamp50 is provided sterile (sterilized by EtO). Do not use if sterility seal has been tampered with or packaging is damaged. | ||
| Line 26: | Line 27: | ||
*The devices and/or component(s) are not made from natural rubber, latex free. | *The devices and/or component(s) are not made from natural rubber, latex free. | ||
====CONSIDERATIONS==== | |||
Stopping hemorrhage as soon as possible is the highest priority in patient management when confronted with significant hemorrhage. Follow standards of care regarding management of bleeding and the sequence in which patient interventions are performed. The normal sequence for hemorrhage control in the field is: | Stopping hemorrhage as soon as possible is the highest priority in patient management when confronted with significant hemorrhage. Follow standards of care regarding management of bleeding and the sequence in which patient interventions are performed. The normal sequence for hemorrhage control in the field is: | ||
*Body Substance Isolation (gloves, eye shields, gowns, as required by policy/procedure) | *Body Substance Isolation (gloves, eye shields, gowns, as required by policy/procedure) | ||
| Line 35: | Line 36: | ||
*Transport patient to appropriate facility for definitive care of wounds | *Transport patient to appropriate facility for definitive care of wounds | ||
====EQUIPMENT==== | |||
*iTClamp50 device (as many as needed to achieve hemostasis) | *iTClamp50 device (as many as needed to achieve hemostasis) | ||
*Standard gauze for cleaning around wound to verify effective application | *Standard gauze for cleaning around wound to verify effective application | ||
*Packing material (as needed to fill large wound cavities) | *Packing material (as needed to fill large wound cavities) | ||
====PROCEDURE==== | |||
[[File:iTClamp_Closing.jpg|thumbnail|iTClamp Closing on Wound]] | |||
If the patient is conscious, explain procedure | If the patient is conscious, explain procedure | ||
*Apply non-sterile gloves | *Apply non-sterile gloves | ||
| Line 61: | Line 63: | ||
**The iTClamp50 may be placed on a wound that has a hemostatic agent applied. The hemostatic agent does not have to be removed. | **The iTClamp50 may be placed on a wound that has a hemostatic agent applied. The hemostatic agent does not have to be removed. | ||
====TRAINING==== | |||
*Hold the device by the gripping bars, press the device further closed to release the lock | *Hold the device by the gripping bars, press the device further closed to release the lock | ||
*While maintaining this pressure on the arms, press both release buttons with your other hand. | *While maintaining this pressure on the arms, press both release buttons with your other hand. | ||
| Line 67: | Line 69: | ||
*Pick up the device ONLY by the buttons to prevent accidental contact with needles | *Pick up the device ONLY by the buttons to prevent accidental contact with needles | ||
*Dispose of the device in accordance with local guidelines for biohazard sharps. | *Dispose of the device in accordance with local guidelines for biohazard sharps. | ||
[[Category:Procedure Guidelines|0938]] | |||
Latest revision as of 17:49, 1 February 2018
Procedure Guidelines
9.38 iTClamp Hemorrhage Control System
TRAINING
iTClamp™ hemorrhage control system requires specific training prior to use.
INDICATIONS
The ITClamp™50 device is a trauma clamp device for the temporary control of severe bleeding in the extremities, axilla and inguinal areas.

CONTRAINDICATIONS
The ITClamp50 is contraindicated where skin approximation cannot be obtained (for example, large skin defects under high tension).
WARNINGS
- This device is intended for temporary use only; FDA Approval is for 24 hours
- Patients must be seen promptly by medical personnel for device removal and surgical wound repair
- Only use device as directed to avoid needle stick injury
- Do not use where delicate structures are near the skin surface, within 10 mm, such as the orbits of the eye
- Will not control hemorrhage in non-compressible sites, such as the abdominal and chest cavities
- Ensure personal protective equipment is utilized to protect against potential splashing of blood during application
PRECAUTIONS
- Single-use, disposable device; not for reuse. Re-use of contents may cause cross contamination, leading to patient risk and complication(s). Can be repositioned.
- iTClamp50 is provided sterile (sterilized by EtO). Do not use if sterility seal has been tampered with or packaging is damaged.
- Not compatible with Magnetic Resonance Imaging (MRI) procedures.
- Dispose of the device in accordance with local guidelines for biohazard sharps.
- The devices and/or component(s) are not made from natural rubber, latex free.
CONSIDERATIONS
Stopping hemorrhage as soon as possible is the highest priority in patient management when confronted with significant hemorrhage. Follow standards of care regarding management of bleeding and the sequence in which patient interventions are performed. The normal sequence for hemorrhage control in the field is:
- Body Substance Isolation (gloves, eye shields, gowns, as required by policy/procedure)
- Initiate direct pressure with a gloved hand once bleeding wound is identified
- Assess significance of bleeding wound, capillary bleeds or very slow flowing hemorrhages may be effectively controlled with directed pressure and standard gauze dressings
- If direct pressure will not be effective (either due to hemorrhage rate or lack of resources), apply the iTClamp(s) as described below
- For extreme extremity injuries not amenable to iTClamp application (e.g. the skin edges cannot be approximated) consider tourniquet application in accordance with policy and procedure.
- Transport patient to appropriate facility for definitive care of wounds
EQUIPMENT
- iTClamp50 device (as many as needed to achieve hemostasis)
- Standard gauze for cleaning around wound to verify effective application
- Packing material (as needed to fill large wound cavities)
PROCEDURE
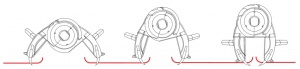
If the patient is conscious, explain procedure
- Apply non-sterile gloves
- Bring package into close proximity to patient wound
- Open the sterile package by pulling forward on the outer tabs
- Remove the device from the package by lifting up, taking care to not close the device until it has been applied to the wound
- If the device has been inadvertently closed, push the side buttons inward with one hand, and pull the device open (using the device arms, do not slide fingers beyond the safety bar)
- Locate the wound edges
- Align the device parallel to the length of wound edge. Position the needles approximately 1-2 cm from the wound edge on either side.
- For very large wounds the device can be applied to one side of the wound first and then pulled over to the other side, or the tissue can be approximated by hand and then the iTClamp applied.
- Press the arms of device together to close the device. Device safety seal will break with pressure.
- Ensure the entire wound is sealed and bleeding stops, using your gauze pad to wipe the area to verify no leaking of blood from the wound.
- A gauze or compression wrap can be placed around the device on the wound to protect the device and increase the pressure on the wound to limit hematoma expansion.
- NOTE: More than one device may be required for large wounds.
- If bleeding continues:
- If the device is in the correct position (pressure bars parallel to the wound edges), close the device more firmly by applying further pressure to the arms of the device.
- If bleeding continues because the wound is too large: apply a second device (or as many devices as needed) to the open section.
- If bleeding continues because the device is not positioned correctly, remove the device according to instructions and reapply.
- If desired, wound packing may be done before the iTClamp50 is placed on the wound
- The iTClamp50 may be placed on a wound that has a hemostatic agent applied. The hemostatic agent does not have to be removed.
TRAINING
- Hold the device by the gripping bars, press the device further closed to release the lock
- While maintaining this pressure on the arms, press both release buttons with your other hand.
- While pressing the release buttons, pull one of the gripping bars open and rotate the needles out of the wound, one side at a time.
- Pick up the device ONLY by the buttons to prevent accidental contact with needles
- Dispose of the device in accordance with local guidelines for biohazard sharps.